The treatment of hydrocephalus often involves the placement of a shunt catheter into the cerebrospinal ventricular space, though such ventricular catheters often fail by tissue obstruction. While diverse cell types contribute to the obstruction, astrocytes are believed to contribute to late catheter failure that can occur months after shunt insertion. Using in vitro microfluidic cultures of astrocytes, we show that applied fluid shear stress leads to adecrease of cell confluency and the loss of their typical stellate cell morphology. Furthermore, we show that astrocytes exposed to moderate shear stress for an extended period of time are detached more easily upon suddenly imposed high fluid shear stress. In light of these findings and examining the range of values of wall shear stress in a typical ventricular catheter through computational fluid dynamics (CFD) simulation, we find that the typical geometry of ventricular catheters has low wall shear stress zones that can favour the growth and adhesion of astrocytes, thus promoting obstruction. Using high-precision direct flow visualization and CFD simulations, we discover that the catheter flow can be formulated as a network of Poiseuille flows. Based on this observation, we leverage a Poiseuille network model to optimize ventricular catheter design such that the distribution of wall shear stress is above a critical threshold to minimize astrocyte adhesion and growth. Using this approach, we also suggest anovel design principle that not only optimizes the wall shear stress distri-bution but also eliminates a stagnation zone with low wall shear stress, which is common to current ventricular catheters.
Papers:
Lee, S., Kwok, N. W., Holsapple, J., Heldt, T., Bourouiba, L. (2020) Enhanced wall shear stress prevents obstruction by astrocytes in ventricular catheters. Journal of the Royal Society Interface. 17:20190884. PDF and SI.
Bourouiba, L., Lee, S., and Heldt, T., (2019) Cerebrospinal fluid space draining catheters. Provisional US Patent - serial number 62/937,975.
Figures shown below:
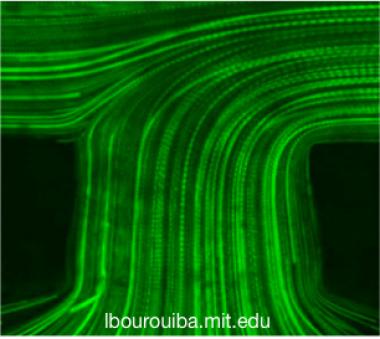
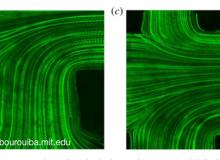
(Left) Flow visualization using fluorescent particle tracking by high-speed microscopy in catheter. (b,c) Pathlines of fluorescent particles illustrate the entry of a flow, followed by the confluence of flows in the lumen. In both (b,c), the Reynolds number is 0.5. The plane of focus of the microscope objective was located to the middle plane of the catheter. Scale bar in (c) is 200 μm and also applies to (b).
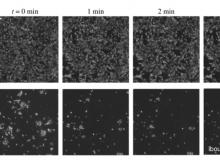
(Right) Phase contrast imaging of the response of astrocytes to short high fluid shear stress of 30 mPa at T = 72 h. Here (above each column), t is the duration for which the high fluid shear stress is imposed. Astrocyte cells cultured under continuous long-term moderate fluid shear stress (b) exhibit more detachment, while those from static culture (a) only show change in cell morphology, with noticeable regions of cell retraction. Scale bar is 200 μm.